I read this story in the newspaper this morning. You are the patient they are talking about; you had HSCT, GVHD and IFI. It was Aspergillus that ultimately killed you.

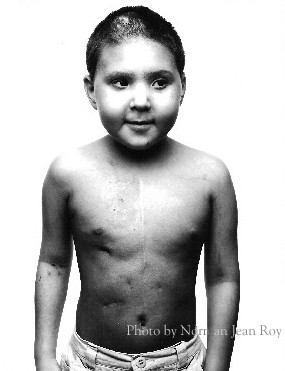
This is the photo that was in the news story. It must be Aspergillus. It is weird to see what the fungus that killed you really looks like. I guess it exploded all through your body when you had sepsis.
But nothing is ever as simple as it seems. They were probably testing the medicine mentioned in the story way back when you had GVHD. It takes a long time to get a new drug approved. Maybe they even tried it on you. I don't remember anymore. Who knows.
All your doctors will be in town next month for a Fanconi anemia scientific meeting, and I should see them then. I signed up to go. Not exactly sure why, but it is good to stay connected. I guess I will ask Dr. Gillio or Dr. Wagner about this when/if I see them.
Dr. Hughes is here in Washington this week for a conference. Mom was invited to go to that but I am not sure she is going to make it. We heard some good news from a family he is helping whose kid has Fanconi. Hopefully everything will work out okay. I am keeping my fingers crossed.
Even though I am probably thinking too simplistic about this Noxafil thing - I still am not feeling too good right now. It all comes down to timing. Bad timing in our case. Clearly, you were born and transplanted (and died) too early.
I was thinking if I wrote a book it would be called "Everything Leads Back to You." There are obvious things like this story and on Sunday when I went to an event and saw your old pediatrician, Giorgio Kulp, and then some rabbi talked about someone who needed a bone marrow transplant (he was telling a story about Jay Feinberg, who not only lived but was very helpful when we were looking for a match for you), but also things that I see or experience everyday that seem totally unrelated to you. But to me they trigger a memory that leads back to you.
I like that. Not a day goes by without thinking about you. A picture of the two of us is on my nightable next to my lamp, so your face is the last thing I see before I turn out the light.
Noxafil Treats Invasive Fungal Infections
Schering-Plough Corporation today reported that the U.S. Food and Drug Administration (FDA) has approved NOXAFIL(R) (posaconazole) Oral Suspension for prophylaxis (prevention) of invasive Aspergillus and Candida infections in patients 13 years of age and older who are at high risk of developing these infections due to being severely immunocompromised, such as hematopoietic stem cell transplant (HSCT) recipients with graft-versus-host disease (GVHD) or those with hematologic malignancies with prolonged neutropenia from chemotherapy. NOXAFIL is the first and only antifungal agent approved by FDA for the prevention of invasive fungal infections (IFIs) caused by Aspergillus species.
Invasive fungal infections most often occur in people who are immunocompromised or immunosuppressed, and are increasingly caused by moulds such as Aspergillus. IFIs are a leading cause of death in these high-risk populations. Patients undergoing hematopoietic stem cell transplant or chemotherapy for hematological malignancies such as acute myelogenous leukemia (AML) or myelodysplastic syndromes (MDS) who develop IFIs have a high mortality rate of 60-90 percent.(1)
The NOXAFIL approval is based on results of two head-to-head randomized clinical studies, the largest prophylaxis studies conducted to date in these high-risk patient populations. A total of more than 1,200 patients were enrolled in these studies, which demonstrated substantially fewer breakthrough Aspergillus infections in these patients. In high-risk neutropenic patients, prophylaxis with NOXAFIL was associated with decreased all cause mortality versus the comparator drugs.
"NOXAFIL can help prevent patients from developing life-threatening invasive fungal infections while being treated for serious conditions, such as acute leukemia or graft-versus-host disease," said John Perfect, M.D., Professor, Department of Medicine, Division of Infectious Diseases, and Director, Duke University Mycology Research Unit. "With this FDA approval, NOXAFIL offers physicians an important new therapeutic option for preventing invasive fungal infections in patients at high risk," he said.
"We are very pleased with today's FDA action and what it means for critically ill patients who are at high-risk for acquiring these fungal infections," said Robert J. Spiegel, M.D., chief medical officer and senior vice president, Schering-Plough Research Institute. "The approval of NOXAFIL, a product discovered and developed by Schering-Plough, reinforces our ongoing commitment to improving treatment options for patients facing life-threatening diseases."
Noxafil Treats Invasive Fungal Infections
09.18.06, 12:00 AM ET
MONDAY, Sept. 18 (HealthDay News) -- A new molecular drug designed to prevent fungal infections in post-surgical patients and others with weaker immune systems has been approved by the U.S. Food and Drug Administration.
Schering Corp.'s Noxafil (posaconazole) contains a substance that has never before been approved in the United States, the FDA said in a statement. The drug was approved to prevent infections caused by certain molds and yeast-like fungi called Aspergillus and Candida.
While people with healthy immune systems are normally unaffected by these fungi, they tend to cause invasive infections in people who have had bone-marrow transplants and people with low white blood cell counts, the agency said.
Noxafil's safety and effectiveness were evaluated in clinical trials involving 1,844 people between ages 13 and 82. Common side effects included nausea, vomiting, diarrhea, rash, a drop in blood potassium levels, and in rare cases, problems with heart or liver function.
The drug should be taken with a full meal to allow for adequate absorption into the body, the FDA said.
No comments:
Post a Comment